KEDRAB Is Supplied in Two Physically Different Vial Sizes
Both single-dose vials contain ready-to-use solution with a nominal potency of 150 IU/mL.1

2-mL vial
The 2-mL vial contains a total of 300 IU, which is sufficient for a child weighing 15 kg (33 lb)1
NDC 76125-150-02
10-mL vial
The 10-mL vial contains a total of 1500 IU, which is sufficient for an adult weighing 75 kg (165 lb)1
NDC 76125-150-10
Dosage
Post-exposure prophylaxis consists of a single 20 IU/kg body weight dose of KEDRAB® (Rabies Immune Globulin [Human]) and a full course of rabies vaccine.1
- Administer KEDRAB as soon as possible after exposure, preferably at the time of the first rabies vaccine dose. However, should a delay occur, administer KEDRAB at any time up to and including seven days after the first dose of rabies vaccine. If there is a delay, initiate post-exposure prophylaxis at any time after exposure.1
- Do not exceed the recommended dose of KEDRAB because this can partially suppress active production of rabies virus antibodies. Do not administer additional doses of KEDRAB, even if the antibody response to vaccination is delayed.1
- KEDRAB is strictly for use in wound infiltration and intramuscular injection after all wounds have been properly infiltrated.1
How to Administer KEDRAB
Proper HRIG Administration Is Crucial to Providing Immediate Protection:
KEDRAB should be administered as soon as possible after an exposure to a potentially rabid animal. The recommended dose of KEDRAB is 20 IU/kg body weight. It is strictly for use in wound infiltration and intramuscular injection (IM). DO NOT administer intravenously.1
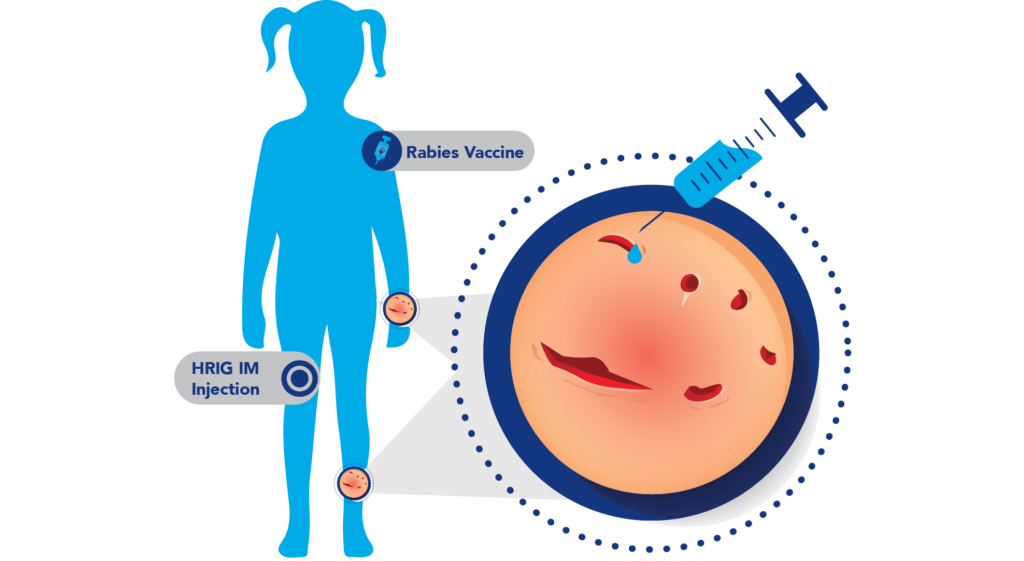
Known Bite Size
If wounds are visible: Bites, scratches, or wounds from a potentially rabid animal such as a dog, cat, raccoon, fox, skunk, or bat.2
- Infiltrate as much of the dose as possible into and around any detectable bite or scratch wounds.1
- Administer any remaining volume intramuscularly into anatomical site(s) distant from the site of the rabies vaccine.1
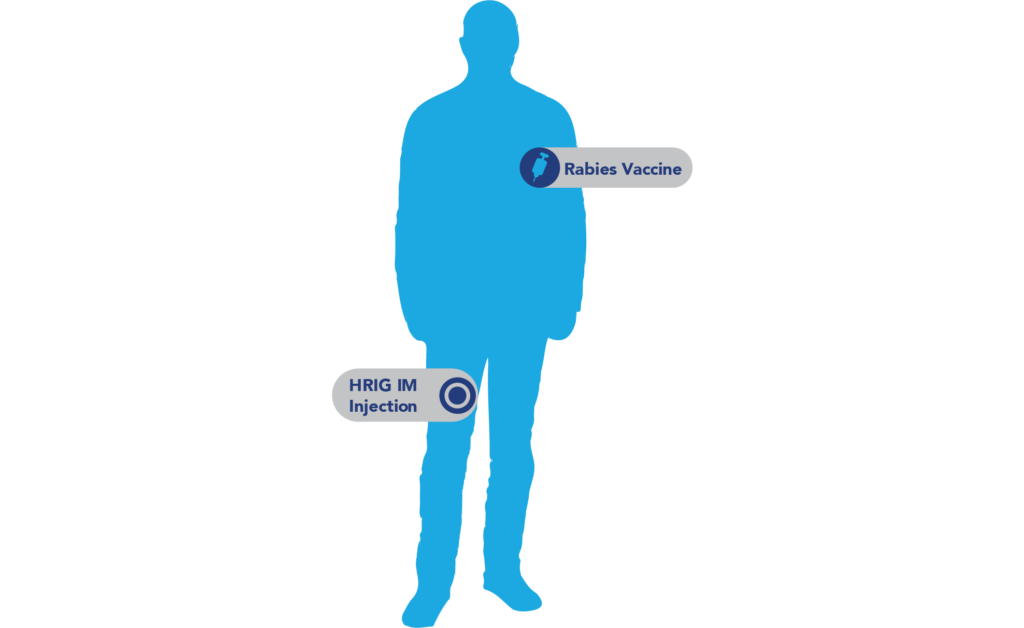
Unknown Bite Size
If wounds are not visible: Undetectable wounds, like a potential exposure to a bat.2
- Administer the full KEDRAB dose intramuscularly at a site distant from the site of the rabies vaccination.1
Please see full Prescribing Information here for full dosage and administration information.
Additional Administration Instructions1:
- If a large intramuscular volume is required (>2 mL for children or >5 mL for adults), administer the total volume in divided doses at different sites.
- DO NOT use the same syringes, needles, and anatomical injection sites for KEDRAB and for rabies vaccine.
- DO NOT exceed the recommended dose of KEDRAB, as this can partially suppress active production of rabies virus antibodies.
- DO NOT administer additional doses of KEDRAB, even if the antibody response to vaccination is delayed.
- Discard unused portion of the HRIG product in the vial.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration. If either of these conditions exists, DO NOT use the vial of KEDRAB; call Kedrion Biopharma Inc. Customer Service (1-855-353-7466). Do not discard the vial.1
Reference: 1. KEDRAB [package insert]. Fort Lee, NJ: Kedrion Biopharma Inc.; 2021. 2. Centers for Disease Control and Prevention. Use of a reduced (4-dose) vaccine schedule for postexposure prophylaxis to prevent human rabies: recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2010;59(2):1-9

