The Centers for Disease Control and Prevention (CDC) and the Advisory Committee on Immunization Practices (ACIP) provide guidance on the safe use of vaccines and related biological products, including post-exposure prophylaxis (PEP) for potential rabies exposures. When administered according to ACIP guidelines, including thoroughly infiltrating all wounds with human rabies immune globulin (HRIG), PEP is essentially 100% effective in preventing human rabies.1
The following support tools provide specific guidance on how to properly administer KEDRAB® (Rabies Immune Globulin [Human]):
KEDRAB Administration Guide

A downloadable pdf that outlines the 3 steps of PEP, according to the CDC and ACIP, and provides step-by-step visual guidance on how to properly administer KEDRAB.
KEDRAB Administration Video
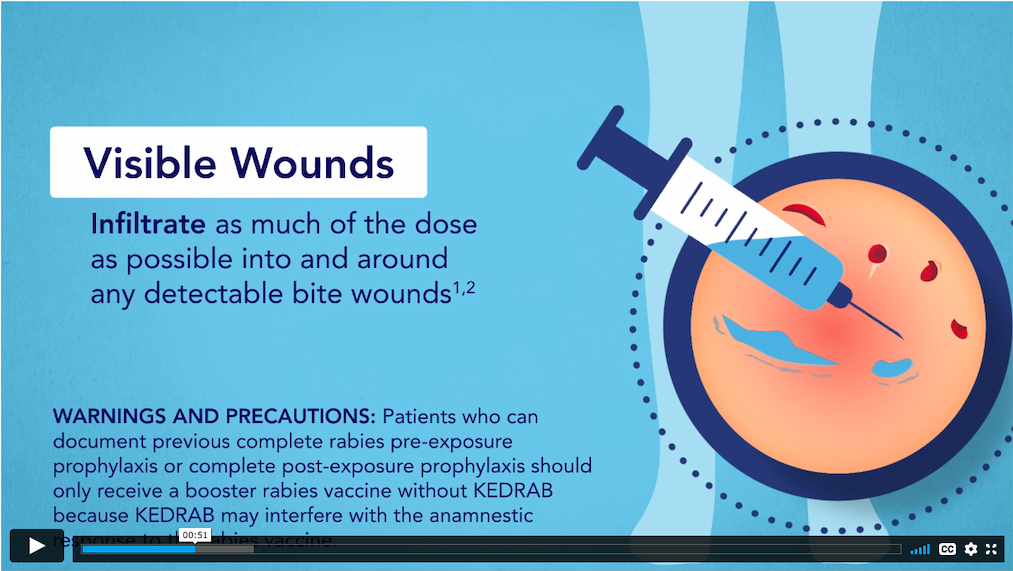
This easy-to-follow animation visualizes detailed instructions on how to properly administer KEDRAB to previously unvaccinated patients.
Need additional information?
Learn more about KEDRAB, Rabies, Ordering, Patient Assistance & Product Replacement Programs, and ways to connect with a Sales Representative.
Reference: 1. Centers for Disease Control and Prevention. Human rabies prevention—United States, 2008: recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2008;57(RR-3):1-28.
