Consideration of HRIG Volume Is Important During Post-exposure Prophylaxis (PEP), Especially When Administering to Children
Read below to learn more about how HRIG volume impacts PEP administration.
PEP Failure Is Often Caused by Administering Insufficient HRIG Volume to Infiltrate All Wounds1
A study evaluating adherence to the CDC/ACIP guidelines on rabies HRIG administration found that1*:
- Only 143 out of 170 patients had clear documentation of the exact volumes of rabies HRIG administered at each anatomical site
- Only 56% of patients received HRIG infiltration into and around the wound (96 of 170 eligible patients with a wound)
*Reasons for PEP failure in this study included improper HRIG infiltration into and around the wound, incorrect administration of HRIG into the buttock, administration of HRIG and rabies vaccine at the same site, and failure to administer HRIG to those with an indication for it.
Study design: This retrospective, cross-sectional study included patients who received at least one dose of rabies IG or rabies vaccine at a multi-hospital health system (one academic medical center, seven community hospitals, and eight additional free standing emergency care centers staffed by board-certified physicians) in Houston, Texas. This study evaluated adherence to CDC–ACIP recommendations for rabies IG patient selection, dosing, timing of administration, and site of administration for rabies postexposure prophylaxis at a multi-hospital health system.1
There Is No Guidance on How to Calculate Minimum HRIG Volume2,3
Determining the proper volume needed for a patient and wound type is required to ensure optimal wound infiltration.2,3
- This can create confusion, since there is another 300 IU/mL HRIG option which reduces dose volume by 50%
- Dosing of any HRIG concentration must have a minimum volume to allow complete infiltration of all wounds, while also adhering to a body weight-based maximum dose of 20 IU/kg
- Determining proper dose volume is crucial when administering HRIG to those with a lower body weight, such as children
Pediatric Use
Globally, 40% of People Bitten by Animals Suspected of Being Infected With the Rabies Virus Are Children Under the Age of 154
Since HRIG dosing is weight-based, a child’s lower body weight will result in less available dose volume to sufficiently treat all scratches and bite wounds.3
Proven 150 IU/mL Potency Ensures a Larger Dose Volume for Complete Wound Infiltration to Help Avoid Post-exposure Prophylaxis (PEP) Failure3,5
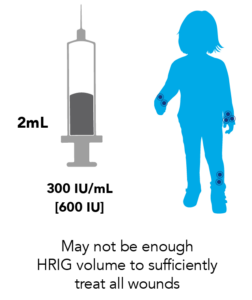
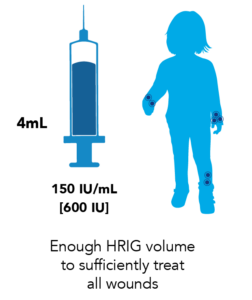
KEDRAB provides adequate volume to infiltrate all wounds5
Compartment Syndrome
Using a Higher Volume of an HRIG Does Not Increase Chances of Compartment Syndrome6
No cases of compartment syndrome were detected in studies measuring the feasibility of RIG wound infiltration in small compartments when performed by experienced healthcare staff following proper post-exposure prophylaxis protocol.6
Reminder: Once all wounds have been properly infiltrated, there is no need to use the remaining HRIG volume in and around the wounds, regardless of concentration.2,5†

HRIG Volume Matters
It is critical that the volume of your HRIG dose is adequate when treating all rabies exposures.
†Any remaining volume should be administered intramuscularly at a site that is distant from the site of the rabies vaccination.
References: 1. Hwang GS, Rizk E, Bui LN, Iso T, Sartain EI, Tran AT, Swan JT. Adherence to guideline recommendations for human rabies immune globulin patient selection, dosing, timing, and anatomical site of administration in rabies postexposure prophylaxis. Hum Vaccin Immunother. 2019;16(1)51-60, doi:10.1080/21645515.2019.1632680. 2. Centers for Disease Control and Prevention. Use of a reduced (4-dose) vaccine schedule for postexposure prophylaxis to prevent human rabies: recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2010;59(2):1-9. 3. Bookstaver PB, Akpunonu P, Nguyen HB, Swan JT, Howington GT. Administration of rabies immunoglobulin: Improving evidence-based guidance for wound infiltration. Pharmacotherapy. 2021 Aug;41(8):644-648. doi: 10.1002/phar.2604. 4. World Health Organization. Rabies. http://www.who.int/mediacentre/factsheets/fs099/en/. Updated April 2020. Accessed December 20, 2023. 5. KEDRAB [package insert]. Fort Lee, NJ: Kedrion Biopharma Inc; 2021. 6. Shantavasinkul P, Wilde H. Postexposure prophylaxis for rabies in resource-limited/poor countries. Adv Virus Res. 2011;79:291-307. doi:10.1016/B978-0-12-387040-7.00013-5.
