Human Rabies Is Essentially 100% Preventable With Proper Post-exposure Prophylaxis (PEP)1
The 3 crucial components of PEP are highly effective in preventing human rabies in exposed patients who have not been previously vaccinated against the virus.2

Clean All Wounds
Immediately cleanse all wounds thoroughly with soap and water, including a virucidal agent2

Administer HRIG for Immediate Protection
Administer a human rabies immune globulin (HRIG), such as KEDRAB, as soon as possible after exposure, but no later than 7 days after the first dose of the vaccine2,3
Administer Rabies Vaccine
Vaccinate against the rabies virus to stimulate the patient’s immune system (virus neutralizing antibodies will appear approximately 7 to 10 days after initiation of the vaccine series)2
Effective PEP Requires Both Passive and Active Immunization2,3
- HRIG provides immediate protection for passive immunization. It is administered only once, and to previously unvaccinated patients, to provide rabies virus-neutralizing antibody coverage until the patient responds to the rabies vaccine.2,3
- The rabies vaccine stimulates the patient’s immune system to produce virus-neutralizing antibodies for ongoing protection, known as active immunization. This occurs approximately 7 to 10 days after initiation of the vaccine series.2,3
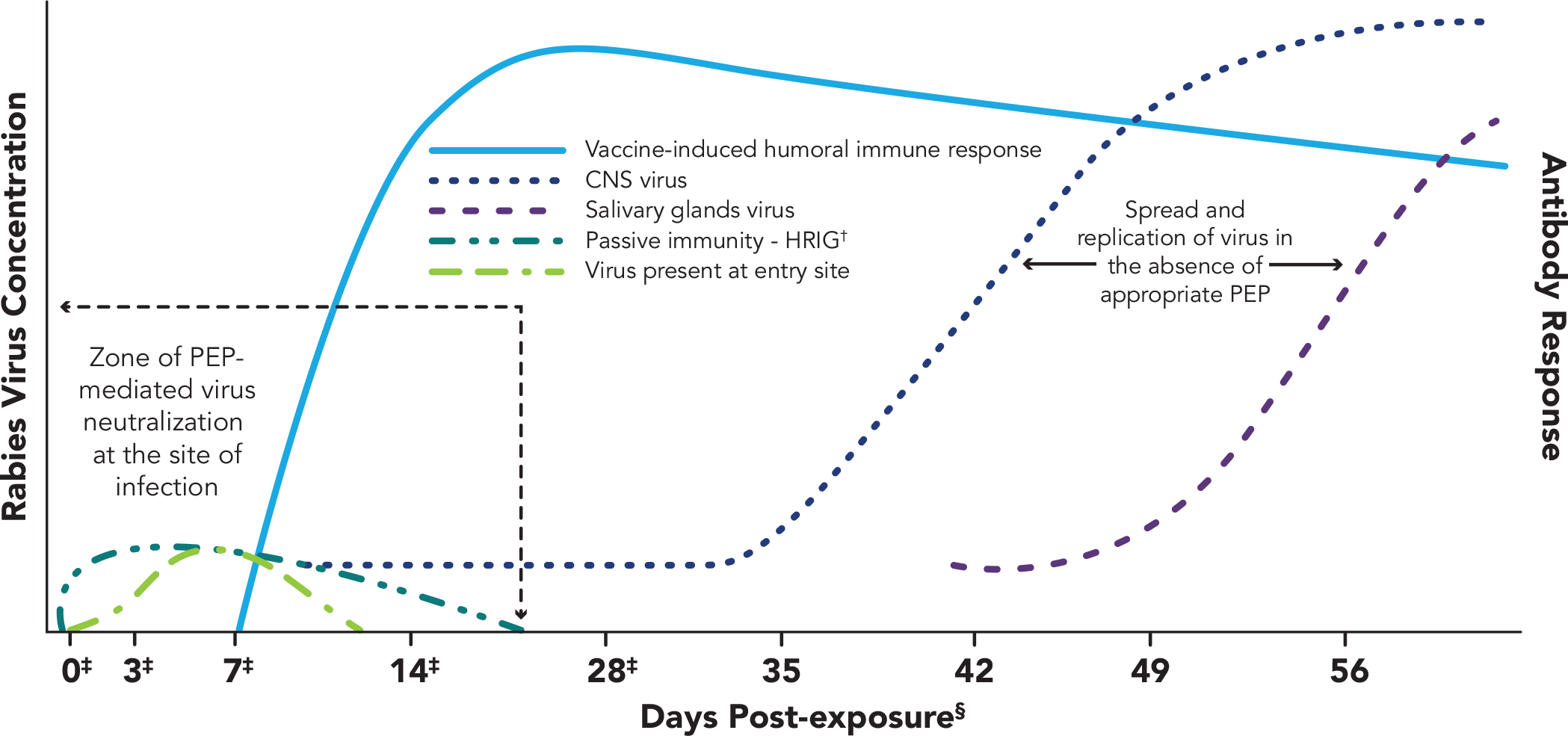
Adapted from CDC Advisory Committee on Immunization Practices 2010.2
*Once in tissues at the entry site, rabies virus can be neutralized by passively administered rabies immune globulin (RIG). Active immunization (vaccine) stimulates the host immune system, and, as a result, virus-neutralizing antibodies (VNA) are produced approximately 7-10 days after initiation of vaccination. By approximately day 14-28 (after administration of 4 vaccine doses), VNAs peak. In the absence of early and adequate PEP, virus enters host neurons, spreads to the central nervous system (CNS), and causes disease, with inevitable fatal consequence.
†Human rabies immune globulin.
‡Day vaccine administered.
§Rabies can progress through five stages: incubation period (5 days to >2 years: US median ~35 days), prodrome state (0-10 days), acute neurologic period (2-7 days), coma (5-14 days), and death.
NOTE: If a patient is immunocompromised, they must receive 5 doses of vaccine on days 0, 3, 7, 14, and 28.2
The Advisory Committee on Immunization Practices (ACIP) Guidelines
PEP for Rabies2 |
||
|---|---|---|
| Vaccination Status | Intervention | Regimen* |
| Not Previously Vaccinated | Wound Cleansing | All PEP should begin with immediate thorough cleansing of all wounds with soap and water. If available, a virucidal agent (eg, povidone-iodine solution) should be used to irrigate the wounds |
| Human Rabies Immune Globulin (HRIG) | Administer 20 IU/kg body weight. If anatomically feasible, the full dose should be infiltrated around and into the wound(s), and any remaining volume should be administered at an anatomical site (intramuscular) distant from vaccine administration. Also, HRIG should not be administered in the same syringe as vaccine. Because HRIG might partially suppress active production of rabies virus antibody, no more than the recommended dose should be administered | |
| Vaccine | Human diploid cell vaccine (HDCV) or purified chick embryo cell vaccine (PCECV) 1.0 mL, IM (deltoid area†) 1 each on days 0,‡ 3, 7, 14 | |
| Previously Vaccinated§ | Wound Cleansing | All PEP should begin with immediate thorough cleansing of all wounds with soap and water. If available, a virucidal agent such as povidone-iodine solution should be used to irrigate the wounds |
| HRIG | HRIG should not be administered | |
| Vaccine | HDCV or PCECV 1.0 mL, IM (deltoid area†), 1 each on days 0‡ and 3 | |
Adapted from CDC Advisory Committee on Immunization Practices 2010.2
*These regimens are applicable for persons in all age groups, including children.
†The deltoid area is the only acceptable site of vaccination for adults and older children. For younger children, the outer aspect of the thigh may be used. Vaccine should never be administered in the gluteal area.
‡Day 0 is the day dose 1 of vaccine is administered.
§Any person with a history of pre-exposure vaccination with HDCV, PCECV, or rabies vaccine adsorbed (RVA); prior PEP with HDCV, PCECV, or RVA; or previous vaccination with any other type of rabies vaccine and a documented history of antibody response to the prior vaccination.
NOTE: For persons with immunosuppression, rabies post-exposure prophylaxis should be administered using all 5 doses of vaccine on days 0, 3, 7, 14, and 28.2
PEP Failure May Be Caused by Improper HRIG Administration2,4,5:
If the 3 components of PEP are not followed promptly and properly, a patient may not be fully protected and may be at increased risk of death from the rabies virus. Specific to the administration of HRIG, there are several errors that can contribute to PEP failure2,4,5:
- Not administering HRIG at all4
- Only injecting HRIG intramuscularly when wound site(s) are visible4
- Not injecting, or inadequate infiltration of, HRIG into all wounds2,4,5
- HRIG volume not sufficient to treat all wounds4,5
Real-World HRIG Failure
A study evaluating adherence to the CDC–ACIP guidelines found, of eligible patients6:
- 44% did not receive HRIG infiltration into and around the wound
- 17% were incorrectly injected with HRIG into the buttock
- 10% had both HRIG and the rabies vaccine incorrectly administered at the same site
- 9% with an indication for HRIG did not receive it
Failure to infiltrate HRIG into and around eligible wounds was the largest area of non-adherence to guideline recommendations6
Study design: This retrospective, cross-sectional study included patients who received at least one dose of rabies IG or rabies vaccine at a multi-hospital health system (one academic medical center, seven community hospitals, and eight additional free standing emergency care centers staffed by board-certified physicians) in Houston, Texas. This study evaluated adherence to CDC–ACIP recommendations for rabies IG patient selection, dosing, timing of administration, and site of administration for rabies post-exposure prophylaxis at a multi-hospital health system.6

HRIG Volume Matters
It is critical that the volume of your HRIG dose is adequate when treating all rabies exposures.
References: 1. Centers for Disease Control and Prevention. Human rabies prevention—United States, 2008: recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2008;57(RR-3):1-28. 2. Centers for Disease Control and Prevention. Use of a reduced (4-dose) vaccine schedule for postexposure prophylaxis to prevent human rabies: recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2010;59(2):1-9. 3. KEDRAB [package insert]. Fort Lee, NJ: Kedrion Biopharma Inc.; 2021. 4. Wilde H. Failures of post-exposure rabies prophylaxis. Vaccine. 2007;25(4):7605-7609. 5. Wilde H, Sirikawin S, Sabcharoen A, et al. Failure of postexposure treatment of rabies in children. Clin Infect Dis. 1996;22(2):228-232.Health and Disease Blog. Rabies virus: properties, mode of transmission, pathogenesis, types, prevention, vaccination, lab diagnosis. https://www.healthdiseaseblog.com/2013/02/rabies-virus-properties-disease-transmission-pathogenesis-neurotrophicvirus-types-of-rabies-virus-street-virus-fixed-virus-vaccine-lab-diagnosis.html. Accessed October 19, 2020. 6. Hwang GS, Rizk E, Bui LN, Iso T, Sartain EI, Tran AT, Swan JT. Adherence to guideline recommendations for human rabies immune globulin patient selection, dosing, timing, and anatomical site of administration in rabies postexposure prophylaxis. Hum Vaccin Immunother. 2019;16(1)51-60. doi:10.1080/21645515.2019.1632680.
